DURC and PEPP USG Policy
Update 06/02/25: A new Executive Order was issued on May 5, 2025, calling for a pause of dangerous biological research that could pose threats to public health, safety, or national security. This order directs federal agencies to revise or replace the 2024 DURC/PEPP policy within 120 days by the Office of Science and Technology Policy (OSTP). On May 7th, the NIH formally rescinded the 2024 DURC/PEPP Policy (NOT-OD-25-061) and simultaneously issued Implementation Update: Improving the Safety and Security of Biological Research (NOT-OD-25-112), rescinding the proposed DURC/PEPP policy that was set to take effect on May 6, 2025.
The United States Government Policy for Oversight of Dual Use Research of Concern (DURC) and Pathogens with Enhanced Pandemic Potential (PEPP) has been established to provide federal oversight for conducting and managing federally funded life sciences research on biological agents and toxins that may pose risks to public health and safety, agriculture crops or other plants, food security, economic security, or national security. This policy will be in effect on May 6, 2025.
Policy Definitions
Dual Use Research of Concern (DURC): Life sciences research that, based on current understanding, can be reasonably anticipated to provide knowledge, information, products, or technologies that could be misapplied to do harm with no, or only minor, modification to pose a significant threat with potential consequences to public health and safety, agricultural crops and other plants, animals, the environment, materiel, or national security.
Pathogens with Enhanced Pandemic Potential (PEPP): A type of pathogen with pandemic potential (PPP) resulting from experiments that enhance a pathogen’s transmissibility or virulence, or disrupt the effectiveness of pre-existing immunity, regardless of its progenitor agent, such that it may pose a significant threat to public health, the capacity of health systems to function, or national security. Wild-type pathogens that are circulating in or have been recovered from nature are not PEPPs but may be considered PPPs because of their pandemic potential.
Pandemic Potential Pathogen (PPP): Pathogen that is likely capable of wide and uncontrollable spread in a human population and would likely cause moderate to severe disease and/or mortality in humans. Examples include H5N1 influenza viruses, SARS-CoV-2, and MERS.
Reasonably Anticipated: An assessment of an outcome such that, generally, individuals with scientific expertise relevant to the research in question would expect this outcome to occur with a non-trivial likelihood. It does not require high confidence that the outcome will definitely occur but excludes experiments in which experts would anticipate the outcome to be technically possible, but highly unlikely.
Institutional Review Entity (IRE)
The entity established by a research institution to execute institutional oversight responsibilities. At UNC Charlotte, this is a Subcommittee of the Institutional Biosafety Committee (IBC).
Additional Definitions
Biosafety
The application of practices, controls, and containment infrastructure that reduces the risk of unintentional exposure to, contamination with, release of, or harm from pathogens, toxins, and other associated biological materials.
Biosecurity
The application of security measures designed to prevent the loss, theft, misuse, diversion, unauthorized possession or material introduction, or intentional release of pathogens, toxins, biological materials, and related information and/or technology.
Biological Agents
Microorganisms or their components capable of causing harm to living organisms, food, water, equipment, or the environment.
Dual Use Research
Research conducted for legitimate purposes that generates knowledge, information, technologies, and/or products that can be utilized for benevolent or harmful purposes.
Federal funding agency
A federal department, agency, institute, center, or office that funds or sponsors intramural or extramural research at research institutions in the United States or internationally.
Institutional Biosafety Committee (IBC)
The entity at UNC Charlotte charged with responsibility for oversight of research using biological materials that entails potential risk to humans, animals, plants, or the environment.
Institutional Contact for Dual Use Research (ICDUR)
The official designated by UNC Charlotte to serve as an internal resource for application of this Policy as well as the liaison (as necessary) between the institution and relevant federal funding agencies.
Institutional Review Entity (IRE)
The entity established by a research institution to execute institutional oversight responsibilities. At UNC Charlotte, this is a Subcommittee of the Institutional Biosafety Committee (IBC).
Life Sciences
The study or use of living organisms, viruses, or their products, including all disciplines, methodologies, and applications of biology (including biotechnology, genomics, proteomics, bioinformatics, and pharmaceutical and biomedical research and techniques).
Pathogen with Pandemic Potential (PPP)
A pathogen that is likely capable of wide and uncontrollable spread in a human population and would likely cause moderate to severe disease and/or mortality in humans.
Principal investigator (PI)
The individual who bears ultimate responsibility for all activities associated with the conduct of a research study, including compliance with federal, state, and local laws, institutional policies, and ethical principles. This individual remains ultimately responsible even when some aspects of the research are delegated to other members of the research team.
Self-Assessment Required for DURC and PEPP Research
Principal Investigators (PIs) proposing to work with microorganisms (virus, bacteria, fungi, and parasites) and/or biological toxins that require Institutional Biosafety Committee (IBC) oversight must assess if their research is reasonably anticipated to be within the scope of Category 1 or Category 2 Research as set forth in USG DURC/PEPP Policy before work in the laboratory can begin.
The DURC and PEPP Policy identifies two research categories that must be proactively assessed by the PI:
Category 1 Research
Meets the following criteria:
It involves one or more of the biological agents and toxins from a pre-determined list*;
It is reasonably anticipated to result, or does result, in one (1) of the nine (9) experimental outcomes and
* Pre-determined List: all Select Agents and Toxins, Risk Group 4 pathogens, and a subset of Risk Group 3 pathogens.
NOTE: Possession and use of biohazardous agents deemed to fall under Biosafety Level 4 (BSL-4) is prohibited on campus.
Category 2 Research
Meets the following criteria:
Involves, or is reasonably anticipated to result in a PPP
Is reasonably anticipated to result in, one (1) of four (4) experimental outcomes.
Any research meeting the definition of Category 1 and Category 2 is designated as Category 2.
Summary Category 1 and Category 2 Research
| DURC/PEPP Policy Summary | Category 1 Research | Category 2 Research |
| Primary Risk | The research can be reasonably anticipated to provide, or does provide, knowledge, information, products, or technologies that could be misapplied to do harm with no, or only minor, modification to pose a significant threat with potential consequences to public health and safety, agricultural crops and other plants, animals, the environment, materiel, or national security. | The research can be reasonably anticipated to result in the development, use, or transfer of a pathogen with enhanced pandemic potential (PEPP) or an eradicated or extinct pathogen with pandemic potential (PPP) that may pose a significant threat to public health, the capacity of health systems to function, or national security, through the potential accidental or deliberate introduction of a PEPP or an eradicated or extinct PPP into a human population. Category 2 research may also have dual use risks. |
| Types of pathogens in scope | (1) All agents regulated by the USDA and HHS, as listed in 9 CFR 121, 42 CFR 73, and 7 CFR 331. (2) Risk Group 4‡ Pathogens: As outlined in Appendix B of the NIH Guidelines. (3) Subset of Risk Group 3 Pathogens: Excluding HIV, HTLV, SIV, Mycobacterium tuberculosis (and related strains), Clade II MPVX viruses (without Clade I virulence factors), vesicular stomatitis virus, and certain fungal pathogens (e.g., Coccidioides spp., Histoplasma capsulatum). (4) BSL-3 and BSL-4‡ Agents: Biological agents requiring these containment levels per the BMBL, including agents not assigned a risk group in the NIH Guidelines but listed in BMBL guidance. (5) Agents added during future updates to the USG DURC/PEPP Policy.*Category 1 Research is subject to oversight by UNC Charlotte and federal funding agency. | (1) Any pathogen that is modified in such a way that is reasonably anticipated to result in the development, use, or transfer of a PEPP. This includes the development of new PPPs from non-PPPs as well as the enhancement of existing PPPs. (2) Eradicated or extinct PPPs that may pose a significant threat to public health, the capacity of health systems to function, or national security. |
| Types of experimental outcomes in scope | Nine experimental outcomes | Four experimental outcomes |
| Level of federal review | Funding agency review | Funding agency and federal department level review |
Examples Category 1, Category 2 and Non-Category 1/2 Research
Category 1 Research Example – Monkeypox (Mpox) virus
Category 1 Research Example – Yersinia pestis
Category 1 Research Example – Botulinum neurotoxin producing species of Clostridium
Category 2 Research Example – Cornonaviruses
Category 2 Research Example – Seasonal Influenza
Non-Category 1/2 Research Example – Adenovirus Drug Development
How to begin?
PI Assessment and Registration
Before May 6, 2025: Complete the PI Assessment Form for Category 1 (DURC) or Category 2 (PEPP) Research
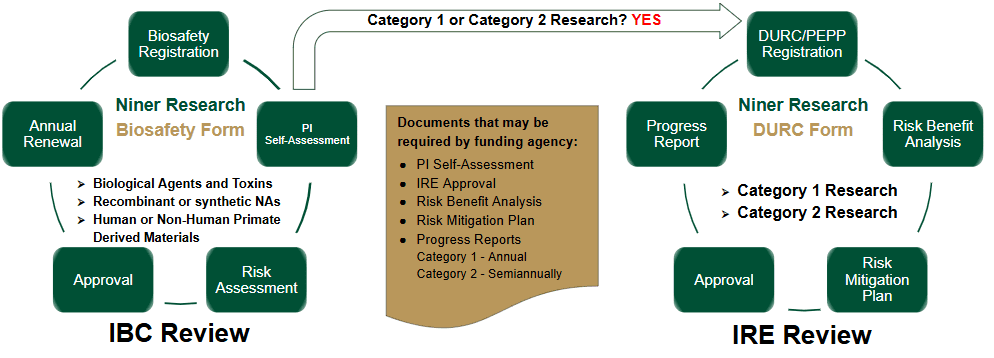
After May 6, 2025: All research conducted or sponsored by UNC Charlotte involving biohazardous materials and/or recombinant or synthetic DNA, and subject to review by the USG DURC/PEPP Policy, must be registered with the Office of Research Protections and Integrity (ORPI) in the Niner Research system. As part of this registration process, PIs are required to complete the self-assessment to evaluate whether their research falls within the scope of Category 1 or Category 2 research under the USG DURC/PEPP Policy. This self-assessment will be reviewed by the Biosafety Program and Institutional Biosafety Committee (IBC) to ensure Institutional compliance.
What Next?
If a protocol meets the criteria for Category 1 or Category 2 Research, the PI must work with the Institutional Review Entity (IRE), a subcommittee of the IBC, to create a risk benefit assessment and risk mitigation plan before the work can begin. This plan, which may include safety enhancements or protocol revisions, must be submitted to the federal funding agency at the proposal stage for review, initial approval, and continuously throughout the research lifecycle (Policy Review Process).
Continuous Review and Oversight
- Biological Materials Initial Registration
- The Principal Investigator (PI) registers their research in the Niner Research system.
- Timeframe: At the beginning of the project, before any research activities start.
- The Principal Investigator (PI) registers their research in the Niner Research system.
- PI Self-Assessment
- The PI completes the self-assessment to determine if the research falls under Category 1 or Category 2 as outlined by the DURC/PEPP policy.
- Timeframe: Immediately after registration and prior to submitting the research proposal.
- The PI completes the self-assessment to determine if the research falls under Category 1 or Category 2 as outlined by the DURC/PEPP policy.
- Risk Assessment
- The Institutional Biosafety Committee (IBC) reviews the PI’s self-assessment and conducts a thorough risk benefit analysis of the research.
- Timeframe: Typically within a few weeks to a couple of months after registration.
- The Institutional Biosafety Committee (IBC) reviews the PI’s self-assessment and conducts a thorough risk benefit analysis of the research.
- Approval
- The IRE, a subcommittee of the IBC, provides approval, including any necessary safety enhancements or protocol revisions. The approved plan is submitted to the funding agency/department for review by the ICUDR.
- After the IRE assessment, and before project initiation. Approval is typically required before funding is awarded.
- The IRE, a subcommittee of the IBC, provides approval, including any necessary safety enhancements or protocol revisions. The approved plan is submitted to the funding agency/department for review by the ICUDR.
- Continuous Monitoring
- The research is continuously monitored for compliance with DURC/PEPP policy during the IBC Annual Review, and any changes in the research may require reevaluation and resubmission for additional review.
- Federal agencies require Category 1 (Annual) and Category 2 (Semiannual) research progress reports to include updates on research progress, key milestones, expenditures, and any challenges faced. Reports must also outline future plans and ensure compliance with relevant regulations.
- Timeframe: Ongoing throughout the entire research lifecycle.
RESOURCES
- NIH Guidelines (April 2024)
- Niner Research – Resource Guide
- DURC/PEPP Policy
- Implementation Guidance
- Select Agent Regulations
- Administration for Preparedness and Response (ASPR) S3 ASPR FAQs
- NIH agency specific info (1/13/2025)
- Institutional DURC/PEPP Program Guideline
- Biosafety Program – Open Forum Session Presentation (April/May 2025)
Need more information? Contact:
Biosafety Program 704-687-1825