Protocols, Amendments, & Renewals
UNC Charlotte requires that a complete animal research protocol be reviewed and approved by the Institutional Animal Care and Use Committee (IACUC) before the investigator obtains live vertebrate animals, or initiates research or teaching involving live vertebrate animals. Animal use in the absence of IACUC approval is a violation of federal laws and University policy.
Investigators are required to consult the Attending Veterinarian prior to submission of new applications and 3-year de novo continuing applications. All IACUC protocols (and protocol renewals and amendments) must be initiated and submitted electronically through Niner Research.
The Office of Research Protections and Integrity (ORPI) performs the initial review of all protocols and amendments.
Click the categories below to learn about protocol submission, special considerations for your application, and the review process for IACUC protocols, annual renewals, and amendments. For detailed information on Pain and Distress, including guidance on classifying planned procedures in animals, refer to the Pain, Discomfort, & Distress page.
PROTOCOL OVERVIEW / NINER RESEARCH
Activities Requiring IACUC Oversight
Before ordering animals or starting any activities with vertebrate animals, including research or educational programs, you must submit a protocol for review and obtain approval from the IACUC. The following types of activities require IACUC review and approval:
- A course using live vertebrate animals in teaching.
- Any research, research training, experimentation, biological testing, or related activity involving live vertebrate animals and conducted at this Institution, in the field under the auspices of UNC Charlotte, or at another institution as a consequence of the sub-granting or subcontracting of a Public Health Service (PHS) conducted or supported activity by this Institution (UNC Charlotte). In this last situation, a Memorandum of Understanding (MOU) is required between the institutions to define the roles of each institution and their IACUCs.
- Any research involving vertebrate animal cadavers from an extramural (non-UNC Charlotte) source.
- Research and teaching activities using tissues derived from live vertebrate animals.
- Field studies (teaching or research) involving any manipulation of any live vertebrate animal or their environment that could be considered stressful (capture/release, trapping, capturing, physical/chemical restraint, invasive procedures, etc.).
Although a UNC Charlotte IACUC protocol is not typically necessary, federal regulations require orders of custom antibodies to be reviewed in advance of order submission. Custom antibodies are those produced in vertebrate animal species using antigen(s) provided by or at the request of an investigator (i.e., not purchased off-the-shelf). If you plan to order custom antibodies, contact the IACUC Coordinator at uncc-iacuc@charlotte.edu with information about your order.
SUBMItting Your protocol application
All new and continuing IACUC protocols, renewals, and amendments of approved protocols must be submitted electronically through Niner Research. Please note that consultation with the Attending Veterinarian is required prior to protocol submission.
To assist you with submission of your Niner Research IACUC protocol, the Office of Research Protections and Integrity / IACUC Office has posted reference guides and video tutorials on the Niner Research Resources Page.
- New protocol applications may be submitted in Niner Research at any time.
- For your protocol to be included on the agenda for an upcoming IACUC meeting for Full Committee Review (FCR), it must be submitted by 12:00 noon on the first day of the month of the scheduled meeting.
- If the deadline falls on a holiday, the protocol should be submitted on the first business day subsequent to that holiday. For more specific information, contact the IACUC Office at 704-687-1872.
If you need further assistance, please contact the IACUC Office (704-687-1872 or uncc-iacuc@charlotte.edu).
IACUC aPPLICATION TYPE
When you initiate a new IACUC protocol application in Niner Research, you will be prompted to select the application type that matches your activity. Basic guidance is provided below and in the protocol application. If you have questions about the appropriate application for your work, please contact the IACUC Coordinator (704-687-1872 or uncc-iacuc@charlotte.edu).
- Experimental Manipulation of Live Vertebrate Animals: If you plan to conduct research, research training, experimentation, biological testing, or related activities in living vertebrates, you will need to submit an Experimental Protocol. This application type should also be selected if you plans to conduct invasive procedures on vertebrate animals in the field.
- Educational Teaching and Observational Studies: You will need to submit an Educational/Observational Protocol for IACUC review and approval PRIOR to field work or the use of animals in teaching lectures or laboratories.
- Using Animal Tissue in Research: If you are obtaining animal tissue from live vertebrate animals from another study either on campus or elsewhere, you need to register your research with the IACUC. Submit a Tissue Protocol for IACUC review and approval PRIOR to the acquisition of animal tissues. If you intend to purchase live animals to be brought to UNC Charlotte in order to euthanize them and collect tissue for research purposes, an Experimental Protocol application must be completed and submitted to the IACUC for review and approval.
- Breeding Animals for Research Purposes: If animals are to be purposely bred for research, a Breeding Protocol must be submitted, approved, and the colony monitored by the IACUC.
- Pilot Studies: A Pilot Study application may be appropriate for novel experimental studies or new techniques, particularly when knowledge of procedures or outcomes is limited. It is also suitable when there is a need to establish endpoints or to determine the feasibility of a study.
Use of Hazardous Materials in Animals
For IACUC protocols that involve use of biological, chemical, or radiation hazards, a concurrent protocol/submission must be approved by the Institutional Biosafety Committee (for biohazards) or the Environmental Health and Safety Office (for chemical and/or radiation hazards). PIs must have these approvals in place BEFORE the IACUC can approve the protocol. All hazardous agents to be used in animals should be listed in Appendix D of the IACUC protocol application.
Consult with the Attending Veterinarian before submitting an application or amendment proposing the use of such agents. Finally, consult with the Office of Research Protections and Integrity (ORPI) / IACUC Office at uncc-iacuc@charlotte.edu or (704) 687-1872 to see if additional compliance or regulatory approvals are needed. PIs should plan submissions accordingly, as a longer timeframe may be required to obtain all necessary approvals.
Steps for IACUC Review and Approval
The IACUC protocol review process involves the following key steps. For detailed information on the review of protocols and amendments, refer to the “Protocol Review” and “Amendments to Approved Protocols” sections below.
- AV Consultation (1-2 weeks): All new and de novo (i.e., continuation) applications, and significant amendments must first be discussed with the Attending Veterinarian (AV) PRIOR to submission to the IACUC. This review is to discuss anesthetics/analgesics, animal care, and any special considerations or requests (e.g., performing research at a USDA pain/distress Category E level or requesting exceptions to regulatory/institutional policies).
- Pre-Review (variable time): Once the protocol application is submitted, it is reviewed by the IACUC Office and again by the AV. This process can take several weeks with multiple requests for clarity and modifications.
- Determination of Level of Review (1 week): Once the protocol passes pre-review, the IACUC determines how the protocol will be reviewed (e.g., review by a Designated Member Reviewer [DMR] or Full Committee Review [FCR] at a convened meeting).
- Review (FCR or DMR): Review can take an additional 2-4 weeks for FCR or 1 week for DMR.
- Revisions: Following review, revisions may be required and subsequent reviews follow until the protocol is approved. Staff members listed must be onboarded, and other relevant approvals (e.g., Biosafety or EHS) must occur prior to IACUC protocol approval.
NOTE: Review times mentioned here are average times and the review may take longer depending on how quickly investigators work with the IACUC and ORPI to resolve questions and respond to review comments. Investigators are asked to carefully consider their research schedule and allow adequate time for the IACUC review. Animal research may not begin until IACUC approval is obtained.
Protocol Approval, Renewal, and Continuation
Unless otherwise specified by the IACUC, protocol approvals are valid for one year and may be renewed annually twice for a total of 3 years of research. Any planned changes in an approved protocol must be submitted via an Amendment Form for prior review and approval. For more information about renewing or amending your protocol, please see the sections titled “Annual Renewals” and “Amendments to Approved Protocols” below.
If a PI wishes to continue a research study beyond the 3-year “life cycle” of IACUC approval, a continuation protocol must be submitted to the IACUC (via Niner Research) for review and approval. All information from the original protocol may be ported into the continuation protocol, with a new protocol number assigned. However, the continuation protocol should be revised to:
- Summarize accomplishments to date
- Include a new literature search for alternatives
- Reflect any changes in procedures, numbers, or activities
- Update the list of personnel
- Update the research goals
PERSONNEL WORKING ON YOUR PROTOCOL
List All Personnel on the Protocol Application
Include all people who will have a role in conducting the research or teaching, such as the PI, research personnel performing procedures on live vertebrate animals (e.g., surgical procedures, tumor/test article injections, genotyping, oral gavage, cage changing, weighing/measuring, etc.), and/or graduate or postdoc teachers.
NOTES:
- There are two pages in the Niner Research protocol application to list personnel. One page is for UNC Charlotte staff and students, and the other is for outside personnel that will be consulting, assisting, doing sub-award work, etc.
- If working with an outside entity / sub-awardee, a Memorandum of Understanding between the IACUCs of both institutions must occur.
Personnel Requirements (before you begin)
The IACUC must determine that all personnel conducting proposed activities are qualified and trained per AWA Regulation 2.31(d)(1)(viii) and PHS Policy C.1.f.
Before an individual is permitted to work with live animals, he or she must complete the following to be deemed “cleared” to engage in animal research or animal care activities:
- Completion of online educational modules via CITI. See the Training and Education page for instructions.
- Completion and submission of an Initial Health History Form, including the most recent tetanus immunization date +/- pre-exposure Rabies prophylaxis (as warranted). Submission of this form enrolls the individual in the Occupational Health Surveillance Program (OHSP). Subsequent clearance by the Occupational Health Professional is required prior to working with animals. See the Occupational Health Surveillance page for forms and instructions.
- Completion of Vivarium orientation training, including an in-person component with the Attending Veterinarian or a Vivarium staff member. Personnel will receive an email invitation to the online Vivarium Orientation course after CITI and Occupational Health requirements are completed. NOTE: Orientation to the Vivarium is only required for individuals listed on protocols that involve work within the Vivarium.
- Inclusion in an IACUC-approved animal care and use protocol as study personnel with specific roles and duties assigned related to the activities proposed.
These requirements apply to ALL faculty, staff, post-doctoral fellows, students, and anyone else handling or exposed to live vertebrate animals or their tissues. Additional details are described in the IACUC’s SOP Requirements for Personnel Listed on IACUC Protocols.
NOTE: Protocol approval will be held until all personnel listed on the application have completed the above onboarding requirements.
Finally, all personnel must be certified as proficient in the techniques to be used in animals. It is the responsibility of the PI to ensure that personnel listed on the protocol receive the hands-on training necessary to achieve proficiency in the selected techniques. The Attending Veterinarian and Vivarium Staff are available for one-on-one consultation and training. Proficiency in a technique may be certified by the Attending Veterinarian, designated Vivarium staff, or the PI.
Considerations for Specific Personnel
Although researchers working with animal tissues may not be in contact with the actual animal (alive or dead), onboarding is still required if the individual is listed on the IACUC protocol. In these cases, individuals must complete the CITI modules “Working with the IACUC” and “Bloodborne Pathogens.” Additionally, enrollment in the Occupational Health Surveillance Program may be necessary, depending on the level of exposure.
For personnel who may only be exposed to or in proximity of animal research being performed in a lab, a quick orientation to the basic animal care/use regulatory environment and potential occupational health risks associated with working in such a lab must be completed prior to beginning work in the lab. Orientation can be arranged through the Office of Research Protections and Integrity (ORPI) / IACUC Office at uncc-iacuc@charlotte.edu or (704) 687-1872.
Visitor, guest, and observer requirements are handled on a case-by-case basis. Such persons must first be cleared by the ORPI / IACUC Office and/or the Vivarium.
THE 3 R’s & LITERATURE SEARCHES
The 3 R’s: Replacement, Reduction, & Refinement
When designing a protocol, a researcher must weigh the benefit to society against the potential pain and suffering of the animals. To mitigate these concerns, the 3 R’s must be considered in the development of the IACUC protocol.
- Replacement: Using alternatives to animal models, such as cell lines, virtual reality, robotics, computer simulations, models/test dummies.
- Reduction: Using the same animals for multiple studies and/or using the minimum number of animals that will yield statistically valid and definitive results.
- Refinement: using better and newer techniques, better instrumentation, improved anesthetic and pain medication regimens, and humane euthanasia at earlier endpoints to minimize pain and distress.
Researchers must also be sure that their proposed research is not duplicative or superfluous. How will it benefit society, man and animals? This must be justified in category D and E studies, but the 3 R’s should be considered for all IACUC protocols.
For more information about how to incorporate the 3 R’s into your research plan, please see:
- National Centre for the Replacement Refinement & Reduction of Animals in Research – The 3Rs
- Using Animals in Scientific Research – Three Rs
- Center for Alternatives to Animal Testing
Literature SearchES
The Animal Welfare Regulations require review of alternatives to procedures that may cause more than momentary / slight pain or distress to the animals. As part of the protocol application, investigators planning Category D or E procedures must provide a written description of the methods used. Additionally, each protocol application must include a literature review that looks for any of the 3 R’s (less painful / distressful alternatives) and determines the availability of alternatives. If such alternatives are found, they should be implemented and the application altered. If these alternatives are not used in the project, then justification must be made in the protocol application and approved by the IACUC.
Literature reviews must be conducted every three (3) years as a part of the renewal / continuation process if a protocol is being renewed for continued work.
When doing a literature review, please use multiple databases. A minimum of two (2) are currently required. Not all databases cover the same information/topics – so more than one is needed.
Also, the keywords and syntax tools you use in your ‘search string’ will generate different articles. Results can be completely different – and potentially incomplete or lacking – if the search string is not constructed correctly.
The USDA National Agricultural Library Animal Welfare Information Center (AWIC) has a step-by-step process online and can also assist with these searches. They will work with you to identify appropriate ‘search strings’ for your research.
NOTE: If you wish for AWIC to conduct the search for you, contact them at the email listed at the bottom of the webpage. They take about 2-4 weeks to complete the search and it is free (as of February 2025, but this is subject to change).
For additional information regarding literature searches, please see:
- Atkins Library at: (704) 687-0491 or via email at askatkins@charlotte.edu
- The USDA National Agricultural Library Animal Welfare Information Center (AWIC) – Literature Searching Animal Use Alternatives
SAMPLE SIZE & STATISTICAL ANALYSIS
A protocol should show how the required number of animals was determined. If this is not done, the IACUC is unable to determine whether the 3 R’s have been met: Reduction, Refinement and Replacement.
The most common method for determining sample size is a power analysis. Calculating the number of animals needed in a statistical power analysis depends heavily on the experimental design. Biological variability and ethical considerations also affect the sample size.
No matter the experiment, when power analyses are used to determine an appropriate number of animals to be used, the protocol should have the following basic elements:
- A clear explanation of the experimental design and the statistical procedure to be used in data analysis,
- A clear description of the trait or traits to be analyzed,
- The expected difference between means of groups (specify which groups and the units for this difference) with a justification,
- The estimated within-groups standard deviation,
- The significance (alpha) level to be used (typically 0.05),
- The power achieved with the estimated number of animals, and
- The software used for the power calculation.
This table provides guidance in determining the sample size needed for a two-sided t test with standard deviation estimated:
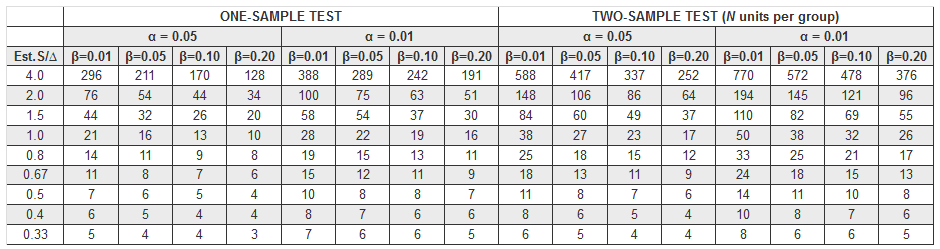
Additionally, you can consult with a statistician, consider submitting a pilot protocol with a small number of animals to see if the research is viable before planning a larger study, or review similar studies for sample sizes to see what is typical.
For further information regarding sample sizes and statistics see:
PILOT STUDIES
For new projects/procedures, projects where outcomes or pain and distress levels are in question, or for Category E Pain / Distress studies for which alternative protocols are deemed necessary but may or may not be suitable, a pilot study should be used. Pilot studies should be used for any new study where outcomes or endpoints are not known or predictable. Typically, pilot studies will be of shorter duration — performed and completed prior to beginning a full study.
Pilot study results can be evaluated and used to justify procedures, the efficacy of alternative procedures, justify the need for a Category E study, or reveal the need to alter procedures or experimentation regimen in the study.
Pilot studies have additional reporting requirements. The purpose of the 90-day report for pilot studies is to inform the IACUC of experimental results and any possible intended/unintended consequences affecting animal health or well-being. The 90-day report serves as a basis for dialog between the PI and Committee on how to address and resolve issues as they arise during experimentation.
The IACUC requires a report from the Principal Investigator 90 calendar days following the initiation of work on the protocol. Subsequent reports may be requested by the IACUC, depending on the review of initial findings.
It is the responsibility of the Principal Investigator to notify the IACUC Office when research activity is about to begin. This will begin the 90-day clock for reporting.
Information to be Included in the 90-day Report
- The original goal of the study (copy/paste from the protocol).
- The total number of animals used to date.
- A summary of experimental results/outcomes thus far.
- A summary of animal health outcomes, including unexpected or adverse events.
- The investigator’s own interpretation of results and outcomes.
- A discussion addressing pertinent changes (if any) in:
- future experiments
- goals of the study
- Documentation regarding whether the study is complete/finished.
- For pilot studies continuing beyond 90 days, the estimated time to completion.
Since animal health information is being requested, it is strongly recommended that PIs consult with the Attending Veterinarian during report preparation to ensure that reported animal health outcomes are in line with veterinary and animal care staff observations.
All reports are to be submitted to the ORPI/IACUC Office, for subsequent distribution to the IACUC. For further information regarding 90-day reports, please see: POLICY: Reporting Requirements for Pilot Studies and Category E (USDA Pain/Distress) Protocols.
PROTOCOL REVIEW
An approved protocol is valid for one year and may be renewed annually twice for a total of three (3) years of study. At the end of three years, a continuation protocol may be submitted to the IACUC. Investigators are notified automatically by Niner Research when deadlines for protocol renewal or continuation are approaching. Review of new and continuing protocols is conducted according to the following process:
Step 1: Initial Review
ORPI/IACUC Staff will conduct an initial review of the application for form completion. The application is forwarded to the Attending Veterinarian (AV) for input and review of animal pain/distress level, proposed procedures and use of analgesics/anesthetics, humane endpoints, method of euthanasia, etc. Within the document, both the ORPI/IACUC Staff and the AV construct a summary of questions or concerns for the Principal Investigator (PI) to address, or request clarifications, if needed, to ensure adequate IACUC review.
NOTES:
- In addition to the AV and the ORPI/IACUC Staff, other Committee members may be asked to “pre-review” the application (e.g., the Environmental Health and Safety representative, the Occupational Health and Safety representative, etc.) and provide feedback to the ORPI.
- The PI can opt to revise and resubmit the application before dissemination to the full Committee or can choose to receive all requests for revision/clarification after the full Committee has reviewed the submission.
Step 2: Determination of Level of Review
The application is distributed to the IACUC for determination of the level of review needed for the protocol (i.e., Designated Member Review or Full Committee Review).
Step 3: Review
Step 3.1: If full committee review is not requested, the Designated Member Review (DMR) process is implemented. In this process, at least one (1) IACUC member, designated by the Chairperson and qualified to conduct the review, reviews the application and has the authority to approve, require modifications to secure approval, or request full committee review of the application.
Step 3.2: If it is decided that the application must go to Full Committee [for] Review (FCR), the application is distributed to the entire IACUC at least one (1) week prior to the Committee’s next convened meeting. The Chairperson assigns a voting member of the Committee to be the lead reviewer of the application and begin discussion of the application at the meeting.
A quorum, consisting of 50 percent of the voting members plus one member, must be present at the IACUC meeting in order to take action on the application. By a vote of the majority of the quorum, the Committee may take the following actions: approve the application, require modifications to secure approval, or withhold approval.
Note: IACUC members must recuse themselves from voting and are restricted from being selected as a reviewer in the DMR review process for protocols where they are named principal investigator, identified in specific roles to perform animal research, or involved in other support activities on behalf of the investigator (i.e., peer reviewer for any associated grants, financial backer for the investigator’s start-up company which supports the experimentation under IACUC review, etc.).
When a protocol is reviewed at a duly convened meeting and it is determined that substantive information is lacking such that a response from the PI is required, the IACUC may take either of the following actions:
- Require modifications to secure approval and require that the revised protocol be returned for FCR at a subsequent duly-convened meeting, OR
- Because all members of the UNC Charlotte IACUC have agreed in advance in writing, the committee may decide by unanimous vote to use DMR subsequent to FCR when modification is needed to secure approval. In this scenario, it is understood that any member of the IACUC may, at any time, request to see the revised protocol and/or request FCR of the protocol.
Step 4: Revisions and Final Determination
Following the convened meeting or DMR review, the IACUC Staff convey any concerns or requests for revision to the PI in a timely manner and manage the revision process thereafter.
The approval date is the date that the designated member or the full committee approves the study. Animal work conducted before this date will be reported to OLAW as a serious noncompliance with PHS Policy.
All experimentation involving the use of hazardous biological, chemical, or radioactive agents in animals will be reviewed by the IACUC in conjunction with concurrent review by other compliance committees/review bodies (i.e., Institutional Biosafety Committee for biohazards, the Environmental Health and Safety Office for chemical or radiation hazards). The IACUC protocol cannot be approved and experimentation utilizing hazardous agents/materials cannot be conducted until the respective compliance committee(s)/review body(ies) has/have reviewed and approved the proposed project.
When conditions for approval have been met and any additional regulatory committee review and approval is in place, the ORPI notifies the investigator, IACUC Chair, and Attending Veterinarian in writing that approval has been granted and the study may begin.
If approval is denied, the ORPI similarly notifies the investigator and provides the Committee’s rationale for denying approval. The investigator can submit a new protocol or file an appeal in writing to the IACUC.
ANNUAL RENEWALS
Unless otherwise specified by the IACUC, protocol approvals are valid for one year and may be renewed annually twice for a total of three (3) years of research. The project may be suspended or terminated if the investigator fails to receive renewal approval by the anniversary of the original approval. Niner Research sends reminders to investigators of the upcoming anniversary and the requirement for submission of a renewal. Annual renewal forms are submitted via Niner Research.
Each annual renewal form is reviewed by a member of the IACUC, and the full Committee is regularly notified of annual renewals approved subsequent to the last convened meeting. Any member may at any time request review of an annual renewal submission at a convened meeting of the Committee.
AMENDMENTS TO APPROVED PROTOCOLS
Any planned revision to an approved protocol must be submitted via an Amendment Form in Niner Research for review and approval. The IACUC must approve the amendment before changes can be implemented. Examples of common amendments include:
- addition/removal of personnel,
- change in procedures,
- change in PI,
- changes in the use of anesthetics/analgesics, or
- addition of animals.
The IACUC distinguishes protocol amendments as either minor or significant/major. See the POLICY: Amendment Classifications and IACUC Review Processes” for a listing of examples of minor and significant amendments.
NOTE: A maximum of three (3) significant amendments per protocol can be submitted for IACUC approval without requiring submission of a new protocol. More than three major amendments requires submission of a new protocol.
Prior to submitting an amendment to the IACUC, the Principal Investigator should consult with the Attending Veterinarian regarding the proposed changes. The Principal Investigator must consult with the Attending Veterinarian regarding potential painful procedures as well as changes in anesthesia, analgesia and euthanasia. The Attending Veterinarian will determine the amendment category on a case-by-case basis.
AMENDMENT REVIEW PROCEDURES
REVIEW OF MINOR AMENDMENTS:
Minor amendments may be reviewed by the Committee Chair and/or the Attending Veterinarian and do not require full committee review unless either party requests it. Amendments to add funding sources (i.e., for congruency review) and amendments that only involve personnel changes may be reviewed administratively by the IACUC Staff.
REVIEW OF SIGNIFICANT (MAJOR) AMENDMENTS:
All significant amendments require full Committee review.
Step 1: Initial Review
All amendments are first checked by the ORPI / IACUC Office staff for form completion. The amendment is forwarded to the Attending Veterinarian (AV) for input and review of the proposed procedures. Both the ORPI and the AV construct a summary of questions or concerns for the Principal Investigator (PI) to address, or request clarifications, if needed, to ensure adequate IACUC review.
The ORPI provides feedback to the PI regarding any edits/requests for clarification or additional information. The PI can opt to revise and resubmit the application before dissemination to the Committee or can choose to receive all requests for revision/clarification after the Committee has reviewed the submission.
Step 2: Determination of Level of Review
The amendment is distributed to the IACUC for determination of the level of review needed. The Committee may select any one of the following: 1) use of the Designated Member Review process, 2) review by a quorum of the full Committee at a convened meeting, or 3) submission of a new protocol.
Step 3: Amendment Review
Step 3.1: If full committee review is not requested, Designated Member Review (DMR) is implemented. In this process, at least one (1) IACUC member, designated by the Chairperson and qualified to conduct the review, reviews the amendment and has the authority to approve, require modifications to secure approval, or request full committee review of the application.
Step 3.2: If the amendment must go to Full Committee [for] Review (FCR), it is distributed to the entire IACUC at least one (1) week prior to the Committee’s next meeting. The Chairperson assigns a voting member of the Committee to be the lead reviewer and begin discussion of the amendment at the meeting. The Committee may take the following actions: approve the amendment, require modifications to secure approval, or withhold approval.
NOTE: A quorum, consisting of 50 percent of the voting members plus one member, must be present at the IACUC meeting in order to take action on the amendment. Approval of significant amendments may only be granted with the approval vote of a majority of the quorum.
Step 4: Revisions and Final Determination
Following the convened meeting or DMR review, the IACUC Staff convey any concerns or requests for revision to the investigator in a timely manner and manage the revision process thereafter.
When conditions for approval have been met and any additional regulatory committee review and approval is in place, the ORPI notifies the Principal Investigator , the IACUC Chair, and the Attending Veterinarian in writing that approval has been granted and the proposed changes may be implemented.
If approval is denied, the ORPI/IACUC Office similarly notifies the Principal Investigator and provides the Committee’s rationale for denying approval. The investigator can submit a new amendment or file an appeal in writing to the IACUC.
ADMINISTRATIVE HANDLING OF SIGNIFICANT AMENDMENTS BY VVC
Per Federal guidance, the IACUC has approved the option of the administrative handling of the following types of significant (major) amendments through Veterinary Verification and Consultation (VVC):
- Changes in anesthesia, analgesia, sedation, or experimental substances,
- Changes in euthanasia to any method approved by the AVMA Guidelines for the Euthanasia of Animals, and
- Changes in duration, frequency, type, or number of procedures performed on an animal.
NOTE: Addition of a new procedure is not eligible for VVC and requires full IACUC review (DMR or FCR).
However, all of the following conditions MUST be met in order for administrative handling (without IACUC review and approval) to be used:
- Attending Veterinarian consultation is documented in writing. The AV, per this guidance will be reviewing proposed changes for the impact they may have on animal pain/distress; AND
- The proposed change does not raise any concerns with the AV (i.e., possibility for potential for increased animal pain/distress, personnel training/proficiency issues, etc.); AND
- The AV can confirm that the change is covered by an IACUC approved policy, guideline, and/or SOP (without deviating from parameters laid out in such documents).
NOTE: The AV has the authority to request that the significant change/major amendment be reviewed and approved by the IACUC (i.e., Designated Member Review or Full Committee Review).
In cases where the types of significant (major) amendments eligible for administrative handling conform to all of the above conditions, the Office of Research Protections and Integrity (ORPI) / IACUC Office can process them once the AV consultation is received.
Please also refer to the Amendment Classifications and Review Processes Policy for more guidance.
ONCE YOUR PROTOCOL IS APPROVED
Ordering animals
You will be asked by the animal care staff to complete an Animal Order Form. Contact the Vivarium at (704) 687-5017 for more information on ordering animals.
Guests, Visitors, or Collaborators: Special considerations before entering the Vivarium
All attempts will be made to honor requests for visits to the Vivarium. Visitors will be asked to sign a logbook and a Memorandum of Understanding that contains assurance statements to which the visitor agrees upon entry. These also inform the individual of possible sequelae to entering the facility. Personal protective equipment (PPE) provided by the Vivarium must be worn when entering procedure rooms or animal holding areas.
Visitors who have been in other animal facilities housing rodents or who have rodents as pets will be asked to refrain from entering the Vivarium for 48 hours after the last exposure.
Contact the Vivarium at (704) 687-5017 for more information or to schedule a visit.
Post-Approval Monitoring
During the three (3) year cycle of an approved protocol, it may be chosen by the IACUC for Post-Approval Monitoring (PAM). This is an onsite review of the protocol, training procedures, hands-on techniques, etc.
For more information regarding this process, please review the Policy: Procedure on Post-Approval Monitoring.