Sample Size & Statistical Analysis
A common reason for delay of protocol approval is inadequate statistical assessment. If the protocol does not show how the required number of animals was determined, the IACUC is unable to determine whether the three Rs have been met: Reduction, Refinement and Replacement.
Calculating the number of animals needed in a statistical power analysis depends heavily on the experimental design to be used. No matter what the experiment, when power analyses are used to determine an appropriate number of animals to be used, the protocol should have the following basic elements:
- A clear explanation of the experimental design and the statistical procedure to be used in data analysis;
- A clear description of the trait or traits to be analyzed;
- The expected difference between means of groups (specify which groups and the units for this difference) with a justification;
- The estimated within-groups standard deviation;
- The significance (alpha) level to be used (typically 0.05);
- The power achieved with the estimated number of animals, and
- The software used for the power calculation.
This table provides guidance in determining the sample size needed for a two-sided t test with standard deviation estimated:
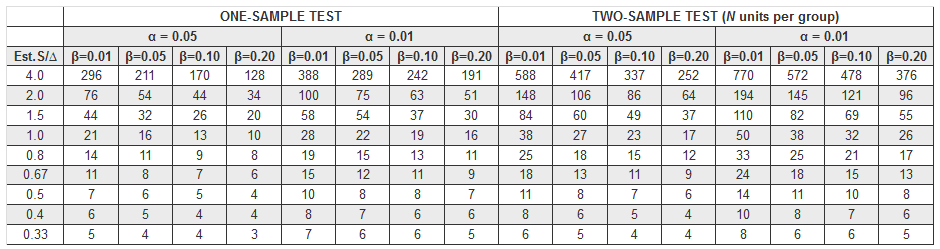
Table from Text: Pharmaceutical Statistics (Practical & Clinical Applications) by Sanford Bolton